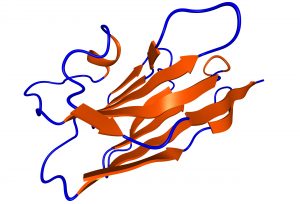
Gulliver Biomed builds further on the expertise acquired in the field of immunomodulation. We aim to foster nanobody research in general and bring these powerful tools to the scientific community and private industry at large.

Nanobodies afford many opportunies to the research community. They can reduce inherent problems of reproducibility experienced with conventional antibodies because their cDNAs are obtained following phage panning and they are 10 times smaller compared to regular antibodies. We can provide you with these sophisticated biomaterials for your biochemical, biophysical, pharmaceutical, cell biological, (medical) imaging research needs. Peruse our catalogue or contact us to develop your own personal set of nanobodies and stay on top of research. As you get accustomed to their flexibility, versatility and broad range of applications in vitro and in vivo, nanobodies will inspire you to envisage new uses and boost your work.